How can boron compounds help to disinfect surfaces?
The interaction of light and matter is one of the most fascinating processes occurring in nature. Chemistry and physics of photoactive compounds are the fields in which these phenomena are particularly interesting. A team of scientists at the Faculty of Chemistry at the Warsaw University of Technology is working on a new concept of designing effective photosensitisers whose main role is to produce reactive oxygen forms to be eventually used in the process of water purification and in medicine.
Singlet oxygen (1O2) is a reactive form of oxygen occurring widely in nature, and due to its high reactivity and no specificity of effect, it is harmful to organisms. The controlled use of its natural properties changes this dangerous chemical into a universal oxidant and facilitates its use both in obtaining and decomposing various chemical compounds, as well as in medicine, particularly in cancer treatment therapy and as a universal, non-invasive disinfectant.
One method to generate singlet oxygen involves utilising photosensitisers which, after light excitation, may transfer excess energy to oxygen molecules in the ground state (naturally occurring in the atmosphere). In this approach, it is necessary that after light excitation the photosensitiser molecule can move into a triplet state, which in turn may interact with the ground triplet state O2. So far, the majority of utilised photosensitisers has based their effect on the use of heavy atom effect in the molecule structure. However, introducing atom I, Pt, Ru, or Pd considerably increases the compound toxicity and its damaging effect on the environment. Additionally, the cost of producing such compounds is extremely high, which significantly reduces the possibility of their practical application.
A new look
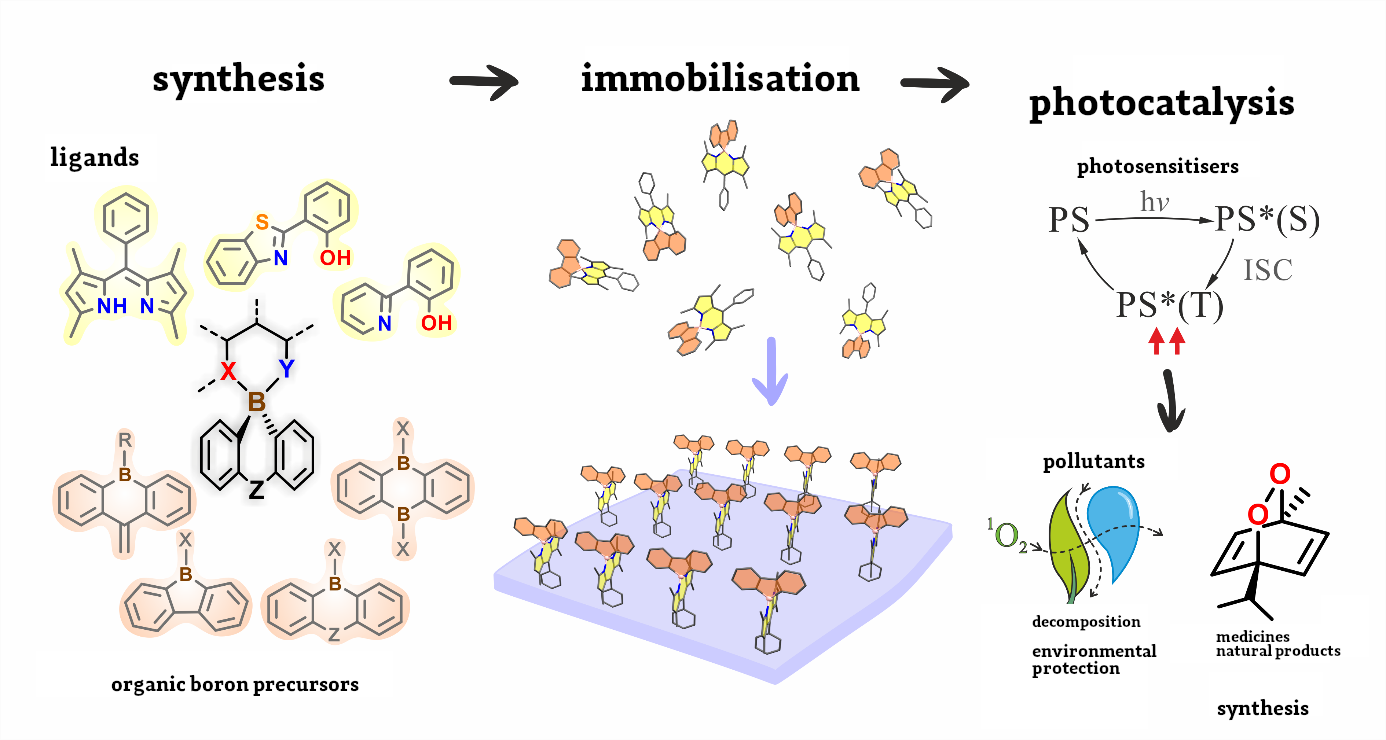
For this reason, most researchers currently focus on purely organic photosensitisers, whose action eventuates from the specific structure of the chemical molecule. However, this requires carefully designed principles of devising such systems, which frequently do not have a universal character, being restricted to some narrow families of chemical compounds. Owing to the simplicity of synthesis and the possibility of controlling the structure and properties of the compound, complex boron compounds seem to ideally fulfil the set requirements of functionality.
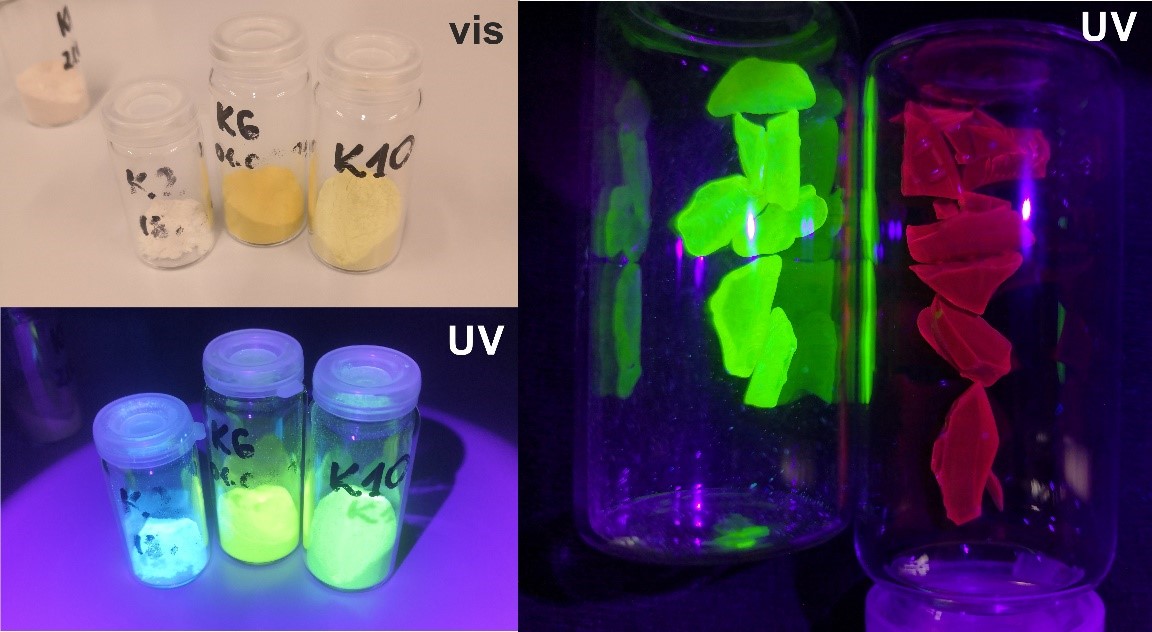
“Complexes of boron compounds with ligands with a coupled bond system have been known for years and extensively analysed in terms of their luminescent properties,” says Krzysztof Durka, Ph.D at the Faculty of Chemistry at WUT. “However, apart from a few exceptions, they do not display any photosensitising properties. My team has adopted an innovative approach to designing this type of photosensitisers. Our research shows that it is crucial to use organic boron complexes with spiro architecture, in which the organic boron part acts as a donor and the ligand acts as a load acceptor, with a boron atom as a natural separator between those entities,” he explains.
This is an innovative approach to this topic. These compounds have never been considered before since researchers focused mainly on introducing changes in the ligand structure only. The proposed strategy is universal and is not restricted to any type of ligand, although dependencies between the electron structure of the compound and its photosensitising properties are clear.
“From the myriad of potential photosensitisers, and there are a few thousand of them, we would like to focus on the most active ones. To find them, it is indispensable to understand the basics of their action and work out the dependence between the compound structure and its properties,” says Paulina H. Marek, BSc, a project participant.
To attain this goal, the team is conducting several quantum mechanical calculations and spectroscopic research whose aim is to find out about the mechanisms behind the generation of triplet states.
Possible applications
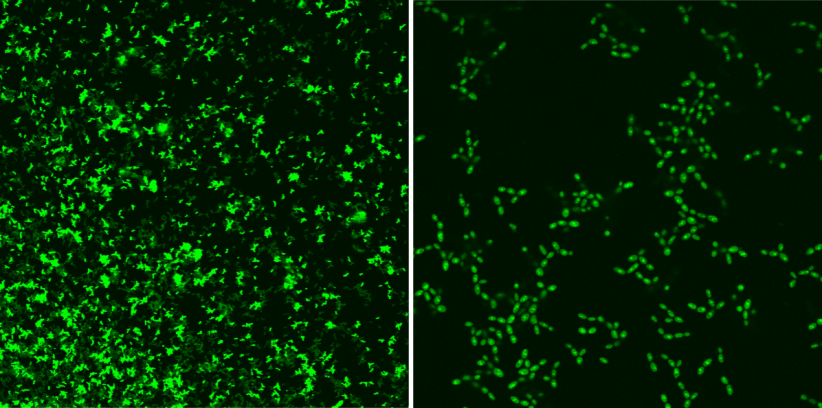
It is worth noting that the optimal parameters of work of the proposed photocatalysts are achieved at merely 0.05 mol% of their content during the conversion of 100% model substrate in 20-120 minutes. The research aims at setting the photoactive system on solid ground. This leads to producing heterogenic catalysts. To test their photocatalytic properties, a special reactor was constructed which facilitates conducting numerous test reactions in a controlled way, with simultaneous exposure to light with a suitable wavelength and constant temperature and airflow maintained. The conducted research gave promising results in terms of the use of the obtained materials in the processes of water purification from pharmaceutical industry pollutants. In addition, the obtained materials undergo anti-microbial tests.
It should be pointed out that singlet oxygen has non-specific effect, a photosensitiser and sunlight are required for its production and its life is very short. It facilitates quick and local neutralisation of bacteria, fungi, and viruses without exposing major organisms to health damage, which is particularly important in the context of the fight against epidemiological threats.
Project „Effective triplet photosensitisers based on organic boron spiro compounds set in the solid ground to be used in photocatalysis” is funded under research grant Technologie Materiałowe-1.
Research team:
Krzysztof Durka, Ph.D; Paulina H. Marek, M.Sc; Karolina Urbanowicz, B.Sc
Source: materials sent by Krzysztof Durka, Ph.D