A new concept of a fuel cell
What springs to your mind when you hear “fuel of the future”? Hydrogen. And how about formic acid? Scientists from the Faculty of Chemical and Process Engineering at WUT are developing a fuel cell powered by formic acid with high stability and increased efficiency.
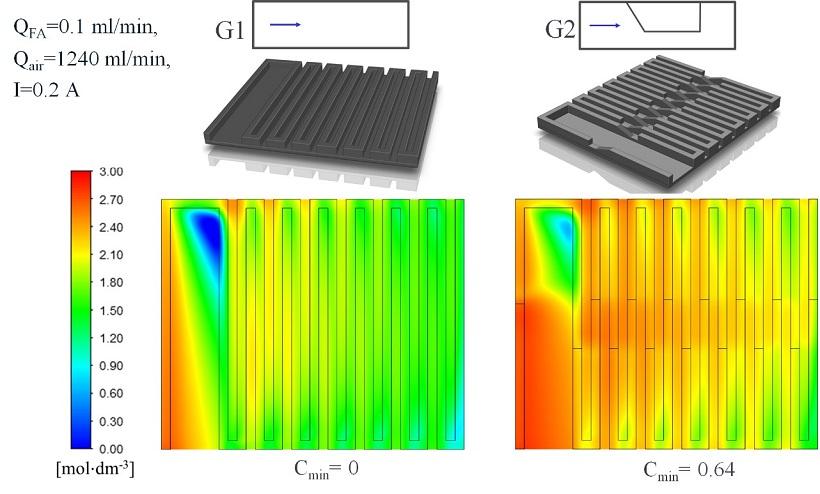
Distribution before and after using turbulising elements, Mass transport enhancement in a direct formic acid fuel cell with a novel channel design, Jałowiecka et al., 2022, https://doi.org/10.1016/j.cej.2022.138474.
Fuel cells are electrochemical devices generating electrical energy from chemical reactions. WUT researchers are working on a low-temperature cell, i.e. working within the range from the temperature of the environment to 120ºC. One of its advantages is, among other things, that it can be frequently turned on and off, as opposed to high-temperature cells.
“The project aimed to improve the cell efficiency by changing the geometry of intake and outtake fuel channels, in this case, formic acid (Direct Formic Acid Fuel Cell, DFAFC), to the catalyser placed in a layer near the catalyser membrane, that is the diffusion layer,” explains Dr Artur Małolepszy from the Faculty of Chemical and Process Engineering at WUT, head of the project.
The selection of formic acid was no accident as this fuel has numerous benefits.
“It is considerably easier to store and transform formic acid due to its liquid form, which facilitates the implementation of such a fuel into the current infrastructure and dissemination of the new technology,” says Monika Jałowiecka, MSc, a doctoral student at FEPCh WUT. “Another advantage is the fact that the volume energy density of formic acid is higher than the volume energy density of hydrogen compressed under the pressure of 70 MPa, which also proves that the energy which we could store and transport for a given volume would be higher if we used formic acid, and the distribution process would be cheaper. Moreover, formic acid may be produced from carbon dioxide in the electro-reduction process ensuring neutral emissions for the fuel cell,” she explains.
Computer simulation
The first stage of work on the new fuel cell would be the modelling of mass transport CFD in the new geometry of channels transporting the substrates. The work on intensifying the reagent transport on the reaction surface was carried out under the supervision of Łukasz Makowski, PhD, DSc. The conducted simulations allowed us to select the most beneficial geometry variant.
“Model simulations show that fuel concentration on the reaction surface halves when the cell is powered with 3-mol formic acid,” says Monika Jałowiecka, MSc. “The reason for this is ineffective diffusion transport, which is slower than convection transport. To increase fuel transport efficiency, we have introduced turbulising elements into the cell, which aim to enforce fuel flow on the anode side and airflow on the cathode side towards the porous electrodes. The solution resulted in considerably improved fuel distribution on the reaction surface and increased efficiency of this cell by eliminating two areas of fuel deficiency in the cell,” she continues.
Additionally, researchers have noticed that turbulising elements support the removal of carbon dioxide bubbles created during the reaction, unblocking the catalyser surface, and reducing voltage losses.
Verification of simulation results
Having the selected type of channels with appropriate geometry, researchers prepared collectors and placed them in the cell to check the data obtained during modelling. Additionally, scientists produced catalysers improving the activity and stability of the link.
“The power and voltage characteristics showed unequivocally that the maximum power of the cell with the new channel geometry with introduced turbulising elements increased even nine times as compared to that obtained in the cell with the standard channel geometry,” says Monika Jałowiecka, MSc. “So far, the maximum growth with divisions noted in the literature has been two-fold only. Such a good result is also a consequence of the difference between the volume energetic density of formic acid and hydrogen. Research on cells powered by formic acid has never been carried out before. Our research fills a cognitive gap, at the same time improving the cell work,” she adds.
Research continues
Currently, WUT scientists are working on the improvement of the cell, both in terms of catalysers and cell structure.
“We constructed and checked a single cell. To power the whole device, we must put a single cell into piles, this way increasing the generated power. Here we come across challenges related to fuel distribution, not only as part of a single cell but also as entire piles,” says Dr Artur Małolepszy.
-
Project “Development of a High Stability and Performance Direct Formic Acid Fuel Cell ” is financed under the research grant POB Energy Conversion and Storage within the “Excellence Initiative – Research University” programme implemented at the Warsaw University of Technology.
Research team:
Dr Artur Małolepszy; Łukasz Makowski, PhD, DSc, associate professor; Marta Mazurkiewicz-Pawlicka, Dsc; Zuzanna Bojarska, MSc; Monika Jałowiecka, MSc.

